Products
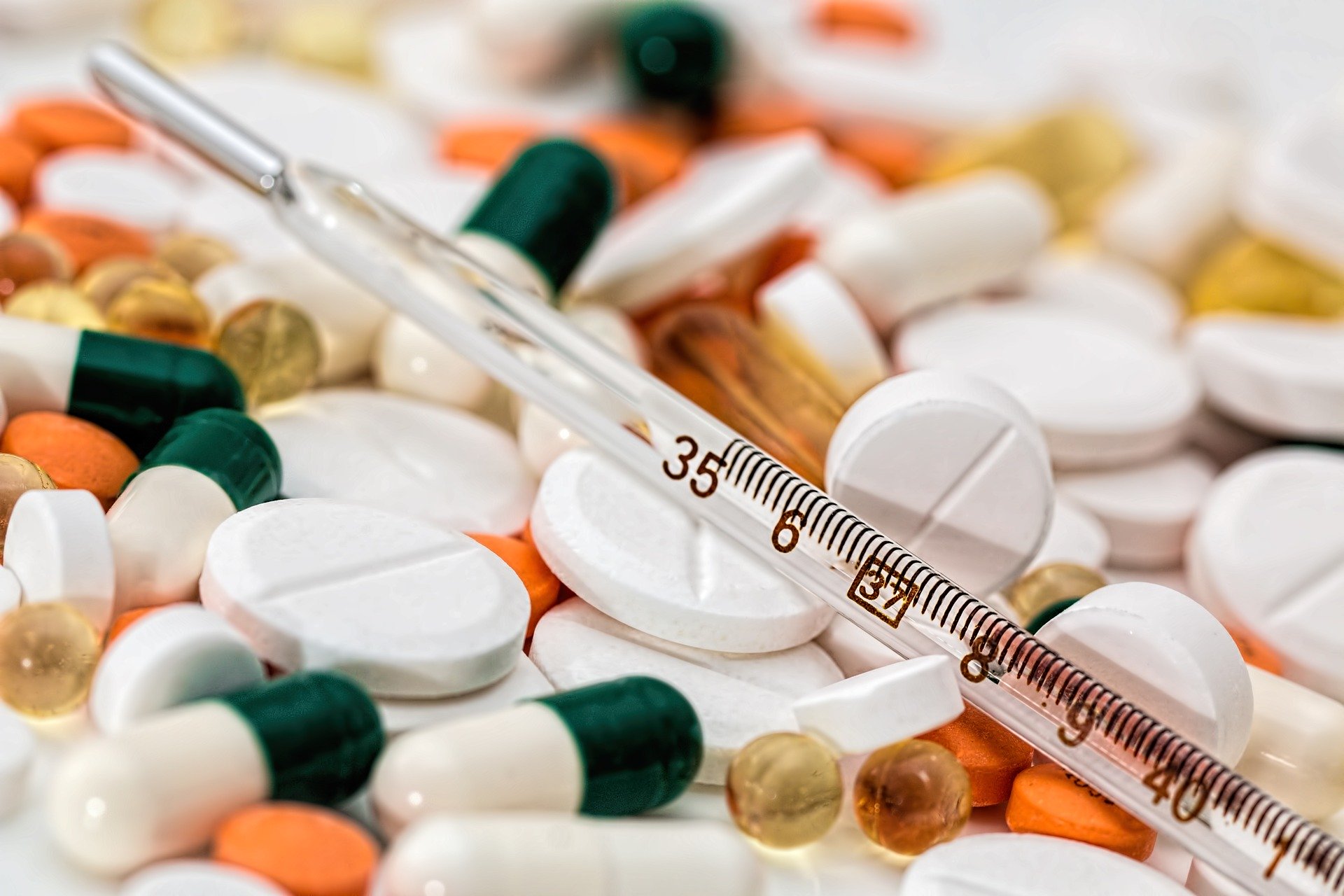
Pharmaceuticals
Segment is dedicated to Generic & Ethical Medicine, our main motive is to provide low cost medicine Worldwide

Cosmetics
Focusing on Skin Friendly Products Specially Chemical Free Products, Also focusing on to provide Herbal Cosmetic

Ayurvedic Products
India is Enriched with Thousands of Herbal Ingredients & which is getting popular Worldwide
Pharmaceuticals
| S.No. | Tablet |
| 1 | Aceclofenac 100 mg uncoated and Film coated tablet.(Aceclofenac Tablet IP) |
| 2 | Aceclofenac (100mg)+ paracetamol (325mg) Film coated tab. |
| 3 | Aceclofenac 100 mg+ Serratiopeptidase 15mg Film Coated Tab. |
| 4 | Aceclofenac 100 mg+Diacerin 50 mg Film Coated Tabs. |
| 5 | Aceclofenac 100 mg+Paracetamol325mg+Chlorzoxazone 250 Film Coated Tabs. |
| 6 | Aceclofenac Sustained release Film coated Tablet 200mg |
| 7 | Aciclovir Uncoated Tab.(200,400mg,800mg). |
| 8 | Albendazole 400 mg Chewable tab. |
| 9 | Albendazole 400 mg+ Ivermectin 6 mg Uncoated tab. |
| 10 | Alfacalcidol (0.25mcg)+Cal.Citrate 1000 mg film coated tab. |
| 11 | Alfacalcidol (0.25mcg)+Cal.co3625mg eq. to calcium 250mg+Betacarotene 2500 IU film coated tab. |
| 12 | Allopurinol 300 mg film coated tablet |
| 13 | Alpha Lipoic acid 100mg+vitamin d3 1000 IU+ Pyridoxine hcl 3 mg+methylcobalamin1500 mcg+folic acid 1.5 mg film coated tablet. |
| 14 | Amitriptyline Hydrochloride IP 10mg, 25 mg Film coated Tablet |
| 15 | Amlodipine Besylate eq. to Amlodipine (5mg) uncoated tab. |
| 16 | Amoxycillin dispersible uncoated tab. 250mg |
| 17 | Artemether (80mg)+ Lumefantrine480mg Film coated tab. |
| 18 | Artemether (80mg)+ Lumefantrine480mg Uncoated Dispersible tab. |
| 19 | Atorvastatin Cal. Eq. to Atorvastatin 10 mg |
| 20 | Atorvastatin Cal. Eq. to Atorvastatin 10 mg+Finofibrate 80 mg/160 mg Film Coated Tabs. |
| 21 | Atorvastatin calcium eq. to Atorvastatin 10mg and Fenofibrate 160mg film coated tablet. |
| 22 | Atorvastatin calcium eq. to Atorvastatin 20mg film coated tablet. |
| 23 | Azithromycin Dihydrate eq. to Azithromycin 250 mg Film coated tab. |
| 24 | Azithromycin Dihydrate eq. to Azithromycin 500 mg Film coated tab. |
| 25 | Betahistine Dihydrochloride IP Uncoated TABLET 8 mg and 16mg |
| 26 | Betahistine Dihydrochloride IP Uncoated TABLET 8 mg and 16mg |
| 27 | Calcium carbonate 1250mg eq. to calcium 500mg+ Vit. D3 2501IU Film coated tab. |
| 28 | Calcium carbonate 1250mg eq. to calcium 500mg+ Vit. D3 2501IU Uncoated tab. |
| 29 | Calcium carbonate 1250mg eq. to calcium 500mg+ Vit. D3 250IU + Zinc sulphate 4 mg+ L-lysine HCl 100mg+Magnesium Hydroxide 50mg Film coated tab. |
| 30 | Calcium citrate 1000 mg + Magnesium100mg + Vit. D3 200 IU + Zinc Monohydrate usp eq. to zinc 4 mg Film coated tab. |
| 31 | Calcium citrate 1000 mg + Magnesium100mg + Vit. D3 400 IU + Zinc Monohydrate usp eq. to zinc 4 mg Film coated tab. |
| 32 | Calcium Citrate malate eq. to calcium250mg +cholecalciferol 100 iu + Folic Acid 50mcg Film coated tab. |
| 33 | Calcium Citrate Tetrahydrate(1000mg) Eq. to Calcium210mg, Vitamin D3 250 IU |
| 34 | Cefixime 200mg + Ofloxacin 200mg Film coated tab. |
| 35 | Cefixime Dispersible tab. 50mg |
| 36 | Cefixime Filmcoated tab. 200mg |
| 37 | Cefixime Trihydrate eq. to cefixime 200mg+ Dicloxacillin Sodium(ER)IP EQ. TO Dicloxacillin 500mg film coated tablet. |
| 38 | Cefixime Trihydrate eq. to cefixime 200mg+ Lactic Acid Bacillus- 2.5 billion spores film coated tablet. |
| 40 | Cefixime Trihydrate eq. to cefixime 200mg+ Lactic Acid Bacillus- 60 million spores film coated tablet. |
| 41 | Cefixime uncoated dispersible tab. 100mg |
| 42 | Cefixime uncoated dispersible tab. 200mg |
| 43 | Cefpodoxime 200mg+ ofloxacin 200mg |
| 44 | Cefpodoxime proxetil eq. to cefpodoxime 100mg uncoated Dispersible tab. |
| 45 | Cefpodoxime proxetil eq. to cefpodoxime 200mg |
| 46 | Cefuroxime Axetil eq. to Cefuroxime 500 mg and 250 mg Film coated tab. |
| 47 | Cetirizine 5mg+Phenylephrine 5 mg+ Paracetamol 325mg uncoated tablet |
| 48 | Cholecalciferol 60000 IU UNCOATED TAB. |
| 49 | Cinnarizine IP 20mg and Domperidone IP 15 mg uncoated tablet. |
| 50 | Ciprofloxacin 500mg,250mg film coated tab. |
| 51 | Citicoline sodium eq. to citicoline 500mg film coated tab. IP |
| 52 | Clarithromycin Tab. 250mg,500 mg Film coated tab. |
| 53 | Clidinium Bromide(2.5mg)+Chlordiazepoxide(5mg)+Dicyclomine HCl(10mg) Film coated tab. |
| 54 | Clonazepam uncoated tablet 0.5 mg |
| 55 | Cotrimoxazole Tablet IP (Trimethoprim IP 160mg and Sulphamethoxazole IP 800mg uncoated tablet.) |
| 56 | Deflazacort uncoated tab. 6mg and 30mg tab. |
| 57 | Diacerein 50mg+ GLUCOSULPHATE Pot. Chloride 750 mg + MSM 250MG Film coated tab. |
| 58 | Diclofenac Potassium (50mg) +Paracetamol (325) + Serratiopeptidase 10 mg Film coated tab. |
| 59 | Diclofenac Potassium (50mg) +Paracetamol (325) + Serratiopeptidase 15 mg Film coated tab. |
| 60 | Diclofenac Potassium (50mg) +Paracetamol (325) Film coated tab. |
| 61 | Diclofenac Potassium 50mg+ Paracetamol 325 mg Uncoated Tab. |
| 62 | Diclofenac Potassium(50mg)+ Serratiopeptidase(10mg) Enteric Coated tab. |
| 63 | Diclofenac Potassium(50mg)+Chlorzoxazone(250mg)+Acetaminophen(325mg) Uncoated tab. |
| 64 | Diclofenac Sodium Film coated Tablet 50 mg |
| 65 | Dicyclomine HCl 10mg uncoated tablet. |
| 66 | Dicyclomine HCl 20mg uncoated tablet. |
| 67 | Diluted Isosorbide Mononitrate 20mg uncoated tab. |
| 68 | Domperidone Dispersible uncoated tab. 10mg |
| 69 | Doxofylline Tablet IP 400 mg uncoated tablet |
| 70 | Doxylamine Succinate(10mg)+Pyridoxine HCl(10mg)+Folic Acid(2.5)mg Enteric Coated tablet. |
| 71 | Doxylamine Succinate(20mg)+Pyridoxine HCl(20mg)+Folic Acid(5mg) Film Coated tablet. |
| 72 | Drotaverine HCl EQ. TO Drotaverine 80 mg + Aceclofenac 100 mg FILM Coated tab. |
| 73 | Drotaverine HCl EQ. TO Drotaverine 80 mg + Mefenamic acid 250mg FILM Coated tab. |
| 74 | Drotaverine HCl EQ. TO Drotaverine 80 mg Film coated tablet. |
| 75 | Drotaverine hcl eq. to Drotaverine 80 mg+ Paracetamol 325 mg Film coated tablet. |
| 76 | Each film coated sustained release tablet contains: Nitrofurantoin IP 100mg |
| 77 | Each film coated tablet contains : Ciprofloxacin Hydrochloride IP EQ. TO Ciprofloxacin 500 mg and Tinidazole 600 mg. |
| 78 | Each film coated tablet contains : Levocetirizine Hydrochloride IP 5 mg , Phenylephrine HCL IP 5 mg and Paracetamol 325 mg. |
| 79 | Each film coated tablet contains : Levocetirizine Hydrochloride IP 5 mg and Ambroxol Hydrochloride 60 mg. |
| 80 | Each Film coated tablet contains : Aceclofenac IP 100 mg and Tizanidine Hydrochloride IP EQ. TO Tizanidine 2 mg |
| 81 | EACH FILM COATED TABLET CONTAINS : Biotin USP 5 mg. |
| 82 | Each Film coated Tablet contains : Calcitriol IP 0.25 mcg, Calcium citrate USP 1000mg, Zinc sulphate Monohydrate IP EQ. TO Elemental zinc 7.5 mg. |
| 83 | EACH FILM COATED TABLET CONTAINS : Desloratadine 5 mg. |
| 84 | EACH FILM COATED TABLET CONTAINS : Diacerein IP 50mg and Aceclofenac IP 100mg. |
| 85 | Each film coated tablet contains : Losartan Potassium IP 25mg and 50mg (Losartan Tablet IP) |
| 86 | Each film coated tablet contains : Losartan Potassium IP 50mg AND Hydrochlorothiazide IP 12.5 mg (Losartan Potassium and HydrochlorothiazideTablet IP) |
| 87 | EACH FILM COATED TABLET CONTAINS : Paracetamol IP 325 mg. and Domperidone IP 10mg |
| 88 | Each Film coated tablet contains : Rosuvastatin calcium IP EQ. TO Rosuvastatin 20 mg.(Rosuvastatin Tablet IP) |
| 89 | Each Gastro-resistant tablet contains : Pantoprazole sodium sesquihydrate IP EQ. TO Pantoprazole 20mg(Pantoprazole Gastro-resistant tablet IP) |
| 90 | Each uncoated dispersible Tablet contains : Cefpodoxime proxetil IP Eq, to cefpodoxime 50 mg. (Cefpodoxime Dispersible Tablet) |
| 91 | Each uncoated dispersible Tablet contains : Levocetirizine Dihydrochloride IP 10mg |
| 92 | Each uncoated tablet contains : Aciclovir IP 200mg (Aciclovir Dispersible tablet IP) |
| 93 | Each uncoated tablet contains : Amlodipine Besilate IP EQ. TO Amlodipine 5 mg and Atenolol IP 50mg |
| 94 | Each uncoated Tablet contains : Ampicillin Trihydrate IP EQ. TO Ampicillin 125 mg.(Ampicillin Tablet IP) |
| 95 | Each uncoated Tablet contains : Cephalexin Monohydrate IP Eq. to cephalexin 125mg(Cephalexin Tablet IP) |
| 96 | Each uncoated tablet contains : Mefenamic Acid IP 250 mg and Dicyclomine Hydrochloride IP 10 mg |
| 97 | Each uncoated tablet contains : Terbinafine HCL TABLET (Terbinafine HCL EQ. to Terbinafine 500mg ) |
| 98 | Each uncoated tablet contains : Thiocolchicoside IP 4MG AND 8MG |
| 99 | Escitalopram Oxalate Eq. to escitalopram 10 mg + Clonazepam 0.5 mg |
| 100 | Esomeprazole Magnesium Tr. Eq. to Esomeprazole 40mg Enteric coated tab. |
| 101 | Etodolac 300mg+ Paracetamol 325mg Film coated tab. |
| 102 | Etodolac 400mg + Thiocolchicoside 4 mg Film coated tab. |
| 103 | Etoricoxib (60mg) Film coated tab.+ Paracetamol 325 mg Film Coated Tablet. |
| 104 | Etoricoxib (90mg) Film coated tab. |
| 105 | Etoricoxib120 mg film coated tablet. |
| 106 | Ferrous Ascorbate eq. To Elemental iron 100mg+Folic Acid1.5mg Film coated tab. |
| 107 | Ferrous Ascorbate eq. To Elemental iron 100mg+Folic Acid1.5mg+Zinc Sulphateeq. To zinc 22.5 mg Film coated tab. |
| 108 | Ferrous Ascorbate eq. to Iron 100mg+ Folic acid 1.1mg+ Methylcobalamin 1.5mg+ Zinc suphate eq. to zinc 22.5mg film coated tablet |
| 109 | Fexofenadine HCl 120mg/180 mg film coated tab. |
| 110 | Fluconazole tab. 150mg uncoated tab. |
| 111 | Fluconazole tab. 200mg uncoated tab. |
| 112 | Folic ACID 5 mg Film Coated tab. |
| 113 | Gabapentin 300 mg + Methylcobalamin 500 mcg Film coated tab. |
| 114 | Glimepiride 2 mg uncoated tab. |
| 115 | Glimepiride 2 mg+ Metformin HCl (SR) 500mg Pioglitazone 15 mg Film coated tab. (BANNED FORMULA) |
| 116 | Glimepiride 2 mg+ Metformin HCl (SR) 500mg uncoated and Film coated tab. |
| 117 | Glimepiride IP 1 mg and Metformin hydrochloride(SR) 1000 mg uncoated tablet |
| 118 | Glimepiride IP 2 mg and Metformin hydrochloride(SR) 1000 mg uncoated tablet |
| 119 | Glimepiride1 mg+ Metformin HCl (SR) 500mg uncoated and Film coated tab. |
| 120 | Haloperidol Tablet 20 mg |
| 121 | Hydrochlorothiazide 25 mg |
| 122 | Hydroxyzine Hydrochloride IP 10mg,25 mg Film coated tablet |
| 123 | Levocetirizine 2.5 mg and Montelukast 4 mg film coated tablet |
| 124 | Levocetirizine HCl 5 MG Film coated tab. |
| 125 | LevocetirizineDiHCL 2.5 mg + Montelukast sodium eq. to montelukast 4 mg Film coated tab. |
| 126 | Levocetirizinehcl(5mg) + Montelukast sodium eq. to Montelukast 10mg Filmcoated tab. |
| 127 | Levocetirizinehcl(5mg) Filmcoated tab. |
| 128 | Levocetirizinehcl(5mg)+PHENYLEPHRINE hcl(5mg)+AmbroxolHCL (30mg)+Paracetamol(325mg) Uncoated tab.(BANNED FORMULA) |
| 129 | Levofloxacin Hemihydrate eq to Levofloxacin 250mg |
| 130 | Levofloxacin Hemihydrate eq to Levofloxacin 500mg |
| 131 | Levothyroxin sodium uncoated tablet IP 25 mcg |
| 132 | Loratadine 10 mg uncoated tab. |
| 133 | Mecobalamin JP-1500 mcg, Folic Acid IP- 5mg, Pyridoxine HCl IP-20mg Film coated Tablet. |
| 134 | Mefenamic Acid 500 mg Filmcoated Tablet. |
| 135 | Metformin HCl (SR) 500 mg film coated and uncoated tablet. |
| 136 | Metformin HCl(500mg)+ Gliclazide(80mg) Uncoated tab. |
| 137 | metformin HCl(500mg)ER+ Glimepiride 1 mg + Pioglitazone 15mg Uncoated tab.(MANUFACTURED UNDER LEAF WARNING ) |
| 138 | Metformin HydrochlorideIP (Sustained Release) 1000 mg Uncoated Tablet |
| 139 | MetforminHCL 1000mg(SR)+ Glimepiride 2 mg Uncoated Tablet. |
| 140 | Methylprednisolone 8 mg , 16 mg uncoated tab. |
| 141 | Methylprednisolone uncoated tab. 4 mg |
| 142 | Metronidazole Tablet Uncoated 400 mg |
| 143 | Montelukast sodium eq. to Montelukast 10 mg |
| 144 | Montelukast sodium eq. to Montelukast 10 mg + Fexofenadine HCl 120 mg Film coated tablet. |
| 145 | Nateglinide(60mg)+MetforminHCL(SR) 500mg Film coated tab. |
| 146 | Nimesulide 100 mg + Serratiopeptidase 10mg Film coated tab.(BANNED FORMULA) |
| 147 | nimesulide 100mg uncoated tablet. |
| 148 | Nimesulide(100mg)+Paracetamol(325mg) uncoated tab. |
| 149 | Nimesulide(100mg)+Paracetamol(325mg)+Tizanidine (2mg) Uncoated tab. |
| 150 | Nitroglycerin 2.6mg |
| 151 | Ofloxacin tab. 200mg , 400mg Film coated tab. |
| 152 | Ofloxacin (200mg)+ Ornidazole(500mg) Film coated tab. |
| 153 | Ofloxacin 200mg+ Nitazoxanide 500mg Film coated tablet.(BANNED FORMULA) |
| 154 | Ofloxacin IP 200MG + CEFIXIME 200MG Uncoated Dispersible Tablet. |
| 155 | Ondansetron orally disintegrating tab. 4 mg |
| 156 | Pantoprazole 40mg and dom 10 mg Gastro-resistant tablet. |
| 157 | Pantoprazole enteric coated tab. 20 mg+ Domperidone 10mg |
| 158 | Pantoprazole enteric coated tab. 40 mg |
| 159 | Paracetamol (325mg)+Aceclofenac (100mg)+ Serratiopeptidase (10mg) Film coated tab. |
| 160 | Paracetamol (325mg)+Aceclofenac (100mg)+ Serratiopeptidase (15mg) Film coated tab. |
| 161 | Paracetamol (325mg)+Dicyclomine HCl(10mg)uncoated tab. |
| 162 | Paracetamol 325 mg + Lornoxicam 4 mg Film coated tab. |
| 163 | Paracetamol 325 mg + Lornoxicam 8 mg Film coated tab. |
| 164 | Paracetamol 325 mg and Dicyclomine HCl 20 mg uncoated tablet |
| 165 | Paracetamol 325 mg+ Diclofenac Potassium 50mg+ Seeratiopeptidase 10mg film coated tablet. |
| 166 | Paracetamol 325 mg+ Diclofenac Potassium 50mg+ Seeratiopeptidase 15mg film coated tablet. |
| 167 | Paracetamol 325mg + phenylephrine HCl 5 mg + Dextromethorphan HBR 10 mg + Cetirizine HCl 5 mg Uncoated tab.(BANNED FORMULA) |
| 168 | Paracetamol 325mg + Tramadol HCl 37.5 mg Uncoated tab. |
| 169 | Paracetamol 325mg+Phenylephrine HCl 5 mg+ Caffeine 30 mg + Diphenhydramin HCl 25 mg Film Coated tablet. |
| 170 | Paracetamol 325mg+Phenylephrine HCl 5 mg+ Caffeine 30 mg + Diphenhydramin HCl 25 mg UnCoated tablet. |
| 171 | Paracetamol 500 mg uncoated tab. |
| 172 | Paracetamol 650mg uncoated tab. |
| 173 | Paracetamol IP 500mg+ Phenylephrine HClIP 5mg + Caffeine IP+ 30 mg + Diphenhydramine HCl IP 25 mg uncoated tab. |
| 174 | Paracetamol IP325mg + Phenylephrine HCl IP 5 mg + Guaiphenesin IP 50 mg + Cetirizine HCl IP 5 mg + Ambroxol HCl IP 15 mg uncoated tablet. |
| 175 | Piracetam 800 mg Film coated tab. |
| 176 | Piroxicam dispersible uncoated tab. 20 mg |
| 177 | Pregabalin(SR) 75mg + Methylcobalamin 1500mcg Film coated tab. |
| 178 | Rabeprazole sodium 20mg enetric coated tab. |
| 179 | Rabeprazole sodium 20mg + Domperidone 10mg enetric coated tab. |
| 180 | Ramipril 2.5 mg + Hydrochlorothiazide 12.5 mg Uncoated tab. |
| 181 | Ranitidine HCl eq. to Ranitidine 150 mg+ Domperidone 10 mg Film coated tablet. |
| 182 | Rosuvastatin tablet IP 10 mg Film coated tablet |
| 183 | Rosuvastatin tablet IP Eq. to Rosuvastatin 5mg, Fenofibrate IP 67 mg Film coated tablet |
| 184 | Roxithromycin 150 mg Film Coated. |
| 185 | serratiopeptidase film coated 10mg |
| 186 | Sildenafil 50,100mg |
| 187 | Slow Diclofenac Tab. BP 75 MG |
| 188 | Sodium Bicarbonate film coated tablet 500 mg |
| 189 | Sodiun Feredetate 231mg+Folic Acid 1.5mg + Vitamin B12 15 mcg Film Coated tablet. |
| 190 | Tadalafil Tablet IP 20 mg film coated tab. |
| 191 | Tapentadol 50mg,100mg Tablet |
| 192 | Telmisartan 40 mg + Amlodipine 5 mg uncoated tab. |
| 193 | Telmisartan 40 mg + Hydrochlorothiazide 12.5 mg uncoated tab. |
| 194 | Telmisartan 40 mg uncoated tab. |
| 195 | Telmisartan IP 40mg + amlodipine 5mg Filmcoated tablet |
| 196 | Telmisartan IP 40mg + amlodipine 5mg uncoated tablet |
| 197 | Terbinafine HCL TABLET USP (Terbinafine HCL EQ. to Terbinafine 250mg ) |
| 198 | Thiocolchicoside (4mg)+Aceclofenac(100mg) Film coated tab. |
| 199 | Thiocolchicoside (8mg)+Aceclofenac(100mg) Film coated tab. |
| 200 | Thiocolchicoside 8 mg + Etoricoxib 60mg Film coated tab. |
| 201 | Thyroxine sodium 25mcg , 50mcg,100mcg. |
| 202 | Tramadol HCl 50mg Uncoated tab. |
| 203 | Tranexamic Acid 500mg + Mefenamic Acid 250mg Film coated tablet. |
| 204 | Trypsin BP 48mg + Bromelain 90mg + Rutoside Trihydrate IP 100mg enteric tablet. |
| 205 | Ursodeoxycholic Acid uncoated tablet 300 mg |
| 206 | Vitamin A IP 10mg, Vitamin B2 IP 5mg,VitaminC 50mg,Vitamin D3400IU,VitaminB1IP10mg,Niacinamide IP 50mg,Vitamin B12IP5 mcg Vitamin E IP 25 IU Film coated Tablet. |
| 207 | Voglibose 0.2mg+Metformin HCl 500mg uncoated tab. |
| 208 | Voglibose Tablet IP, EACH UNCOATED TAB. CONTAINS : VOGLIBOSE IP 0.2 mg. |
| 209 | Voglibose uncoated tab. 0.3 mg |
| 210 | Voglibose uncoated tab. 0.3 mg |
| 211 | Zinc sulphate monohydrate eq. to elemental Zinc 20 mg uncoated tablet. |
| S.NO. | COMPOSITION |
| 1 | Amoxycillin 250mg + Dicloxacillin 250 mg Hard gelatin capsules.(BANNED DRUG) |
| 2 | Amoxycillin 250mg / 500mg capsule. |
| 3 | Betacarotene (30% dispersion)15mg eq. to Vitamin A 7500 IU+ Vitamin E Acetate25mg+vitamin C 100mg+copper 1 mg+zinc 15mg+ selenium dioxide 100mcg+vit.B65mg+Vitamin B12 5 mcg+Folic Acid 1000 mcg Hard gelatin capsule |
| 4 | Calcitriol 0.25mcg + calcium 200mg + zinc 7.50mg Hard gelatin capsule. |
| 5 | Calcium Dobesilate Monohydrate IP 500 mg Hard gelatin capsule. |
| 6 | Carbonyl iron 100mg + Folic Acid 1.5 mg + Vitamin B12 15 mcg + Vit. C 75 mg + Zinc Sulphate 61.8 mg + Selenium 65 mcg capsule. |
| 7 | Cephalexin Capsules IP 500 mg |
| 8 | Clomipramine HCl CAP. IP 50 mg (NA) |
| 9 | Diacerein 50mg Capsule. |
| 10 | Each hard gelatin capsule contains : Alpha Galactosidase 150mg.Lipase Enzyme 3mg,Alpha Amylase Enzyme (1:800)IP50mg, Protease EnzymeUSP 7 mg |
| 11 | Each hard gelatin capsule contains : Clindamycin Hydrochloride IP EQ. TO Clindamycin 300 mg (Clindamycin Capsule IP) |
| 12 | Each hard gelatin capsule contains : Methylcobalamin IP 1500 mcg,Alpha Lipoic AcidUSP100mg,Benfotiamine 50mg,InositolBP 100MG,Folic Acid IP 0.5mg,Chromium Picolinate USP 65mcg,Selenium Dioxide USP 18mcg |
| 13 | Each hard gelatin capsule contains : Rabeprazole Sodium IP 20 mg(As enteric coated pellets) and Diclofenac Sodium IP 100 mg (As sustained release pellets) |
| 14 | Each hard gelatin capsule contains :Pantoprazole sodium IP Eq. to Pantoprazole 40 mg,Levosulpiride 75mg |
| 15 | Esomeprazole Magnesium Tr. Eq. to Esomeprazole 40mg + Domperidone 30mg cap. |
| 16 | FLUCONAZOLE CAPSULE IP 150 mg (Each hard gelatin capsule contains : Fluconazole capsule IP 150mg) |
| 17 | Gabapentin+ Methylcobalamin cap. |
| 18 | Isotretinoin capsule USP (Isotretinoin IP 20 mg Hard Gelatin capsule) (MANUFACTURED UNDER STRICT WARNING) |
| 19 | Itraconazole 100 mg and 200 mg hard gelatin capsule. |
| 20 | Lycopene (10%) 2000mcg + Cyanocobalamin 2.5 mcg + Pyridoxine HCl 1.5 mg + Niacinamide 25 mg + Zinc Sulphate 7.5 mg + Copper Sulphate 1 mg+ Manganese Sulphate 1.5mg+ Betacarotene(10%)10mg + Selenium 25mcg capsule |
| 21 | Methylcobalamin 1500mcg + Calcium 200mg + Calcitriol 0.25mcg + Folic Acid 1.5 mg + Pyridoxine HCl 3 mg Capsule. |
| 22 | Methylcobalamin 1500mcg Capsule. |
| 23 | Methylcobalamin 1500mcg+ Folic acid 1.5mg + Pyridoxine 3 mg + Alphalipoic Acid 100mg+ Selenium 35 mcg + Chromium Picolinate 60 mcg Capsule. |
| 24 | Methylcobalamin 1500mcg+ Folic acid 1.5mg + Thiamine Mononitrate10mg + Pyridoxine 3 mg + Alphalipoic Acid 100mg Capsule. |
| 25 | Omeprazole (20mg) capsule. |
| 26 | Omeprazole (20mg) + Domperidone (10mg) capsule. |
| 27 | Pantoprazole (40mg) + Domperidone(30mg) Capsule |
| 28 | Pregabalin 75mg + Methylcobalamin 750mcg Hard gelatin capsule. |
| 29 | Probiotec and Prebiotic capsule. |
| 30 | Rabeprazole (20mg) + Domperidone(30mg) Capsule |
| 31 | Rabeprazole 20mg + Itopride HCl 150 mg capsule. |
| 32 | Rabeprazole Sodium 20 mg + Levosulpiride 75 mg Hard gelatin capsule. |
| 33 | Rosuvastatin calcium IP 10 mg, Aspirin IP 75mg |
| 34 | Tramadol HCl 50mg Hard gelatin capsule. |
| 35 | Vitamin A 5000 IU with Multivitamins and Multiminerals capsules (Zynac capsule) |
List will be updated soon
| S.NO. | COMPOSITION |
| 1 | Each 5ml Reconstituted Susp. Contains Cefixime Trihydrate eq. to Cefixime 50mg |
| 2 | Each 5ml Reconstituted Susp. Contains Cefixime Trihydrate eq. to Cefixime 100mg |
| 3 | Each 5ml Reconstituted Susp. Contains Amoxycillin Trihydrate eq. to Amoxycillin (Anhydrous) 125mg |
| 4 | Each 5ml Reconstituted Susp. Contains Cefpodoxime Proxetil eq. to Cefpodoxime 50mg |
| 5 | Each 5ml Reconstituted Susp. Contains Cefpodoxime Proxetil eq. to Cefpodoxime 100mg |
| 6 | Each 5ml Reconstituted Suspension Contains : Cehpalexin Monohydrate IP Eq. to Cephalexin Anhydrous 125 mg |
| 7 | Each 5ml Reconstituted Suspension Contains : Artemether IP 40 mg+ Lumefantrine 240mg. |
| 8 | Each 5ml Reconstituted Suspension Contains : Nitazoxanide 100 mg+ Ofloxacin IP 50mg.(BANNED FORMULA) |
| 9 | Each 5ml Reconstituted Susp. Contains Cefixime Trihydrate eq. to Cefixime 50mg + Ofloxacin IP 50mg |
| S.NO. | COMPOSITION |
| 1 | Ciprofloxacine Injection USP 0.2% w/v 100ml |
| 2 | Ciprofloxacine Intravenous Infusion BP 100ml |
| 3 | Compound Sodium lactate Intravenous Infusion BP 100ml, 500ml, 1000ml |
| 4 | Fluconazole Injection USP 100ml |
| 5 | Glucose intravenous infusion BP 25% w/v 100ml, 200ml, 500ml |
| 6 | Glucose intravenous infusion BP 5% w/v 100ml, 200ml, 500ml |
| 7 | Lactated Ringer’s Injection USP 100ml, 500ml, 1000ml |
| 8 | Levofloxacine Intravenous Infusion 100ml |
| 9 | Linezolide Intavenous Injection 600mg/300ml |
| 10 | Mannitol Intravenous Infusion BP 20% w/v 100ml, 200ml, 500ml |
| 11 | Metronidazole Injection USP 0.5% w/v 100ml |
| 12 | Metronidazole Intravenous Infusion BP 100ml |
| 13 | Moxifloxacine Intravenous Infusion 100ml |
| 14 | Ofloxacine Intravenous Infusion 100ml |
| 15 | Paracetamol Infusion 1000mg/100ml, 100ml |
| 16 | Sodium chloride & Glucose Intravenous Infusion BP 0.9% w/v & 5% w/v 100ml, 500ml |
| 17 | Sodium chloride Injection USP 0.9% 100ml |
| 18 | Sodium chloride intravenous infusion BP 0.9% w/v 100ml, 500ml |
| S.NO. | COMPOSITION |
| 1 | Alpha-Beta Artemether Inj. 75mg/ml; 2ml Glass Ampoule |
| 2 | Amikacin Sulphate Injection USP 100mg/2ml, 2ml Glass vial |
| 3 | Amikacin Sulphate Injection USP 250mg/ml, 2ml Glass vial |
| 4 | Amikacin Sulphate Injection USP 500mg/2ml, 2ml Glass vial |
| 5 | Amikacin Sulphate Injection USP 50mg/ml, 2ml Glass vial |
| 6 | Artemether Injection 40mg/ml, 1ml Glass Amlpoule |
| 7 | Artemether Injection 80mg/ml, 1ml Glass Amlpoule |
| 8 | Betamethasone Sodium Phosphate Inj. 4mg/ml; 5ml Glass Ampoule |
| 9 | Chloroquine Phosphate Inj. 40mg/ml; 2ml Glass Ampoule, 10ml Glass Vial |
| 10 | Citicoline Inj. 250mg/ml; 2ml, 4ml, 10ml Glass Ampoule |
| 11 | Dexamethasone Sodium Phosphate Injection USP 4mg/ml, 2ml Glass vial/ Ampoule |
| 12 | Diclofenac Sodium Injection 25mg/ml; 1ml, 2ml, 3ml, 5ml Glass Ampoule |
| 13 | Diclomine HCl Injection 10mg/ml, 2ml Glass Vial |
| 14 | Etophylline 84.7 mg/ml & Theophylline 25.3 mg/ml Inj.; 10ml Glass Ampoule |
| 15 | Gentamicin Sulfate Inj. 40mg/ml; 2ml Glass Vial |
| 16 | Heparin Sodium 1000IU/ml; 2ml Glass Vial |
| 17 | Iron sucrose Injection USP 20mg/ml, 5ml Glass Ampoule |
| 18 | Lidocaine HCl Inj. 10mg/ml; 5ml, 10ml Glass Vial |
| 19 | Methylcobalamin Inj.; 2l, 10ml Glass Vial |
| 20 | Ondansetron Injection 2mg/ml, 2ml, 4ml Glass Ampoule, 20ml Glass Vial |
| 21 | Paracetamol Injection 150mg/ml, 2ml, 5ml Glass Ampoule |
| 22 | Pheniramine Maleate Inj. 22.75mg/ml; 2ml Glass Ampoule |
| 23 | Tobramycine Inj. 40mg/ml; 2ml Glass Vial |
| 24 | Vitamin B-complex Inj.; 5ml, 10ml Glass Vial |
| S.NO. | COMPOSITION |
| 1 | Aceclofenac IP 1.50%w/w,Linseed oil BP 3.00%w/w,Methyl Salicylate IP 10.00%w/w,MentholIP 5.0%w/w,Capsaicin USP 0.01%W/W GEL |
| 2 | Aciclovir cream IP 5% w/w |
| 3 | Adapalene BP 0.10 % w/w and Clindamycin Phosphate IP Eq. to Clindamycin 1 % w/w Gel |
| 4 | Amorolfine Cream (Amorolfine Hydrochloride IP Eq. to Amorolfine 0.25%w/w) |
| 5 | Beclomethasone Dipropionate IP 0.025%w/w and Phenylephrine Hydrochloride IP 0.10%w/w and Lignocaine Hydrochloride IP 2.50%w/w Cream Chlorocresol IP 0.1%w/w AS Preservative |
| 6 | Beclomethasone Dipropionate IP0.025%w/w and Neomycin Sulphate IP 0.5%w/w and Clotrimazole IP1.0%w/w and Chlorocresol IP 0.1%w/w Cream base |
| 7 | Betamethasone cream IP 0.05%W/W |
| 8 | Calamine IP 8% w/v Diphenhydramine HCL ip 1% w/v Lotion.Camphor IP 0.1%W/W |
| 9 | Calamine IP 8% w/w and Liquid Paraffin IP 10% w/w Lotion. |
| 10 | Calcium dobesilate monohydrate IP EQ. TO Calcium Dobesilate 0.25%w/w, Lignocaine HCL ip Eq. to Lignocaine 3.0%w/w,Hydrocortisone Acetate IP 0.25%W/W,Zinc 5.0%w/w cream |
| 11 | Choline Salicylate solution BP Eq. to Choline Salicylate 8.7%w/w and Lignocaine Hydrochloride IP 2.0%w/w Gel Benzalkonium chloride solution IP 0.01%w/w (as preservative)IP 0.01%w/w |
| 12 | Clindamycin and Benzoyl Peroxide Topical gel.(Clindamycin Phosphate IP EQ. TO Clindamycin 1 % w/w and Hydrous Benzoyl Peroxide USP Eq. to Anhydrous Benzoyl Peroxide 2.5 %w/w) |
| 13 | Clindamycin Phosphate eq. to Clindamycin 1 % w/w. |
| 14 | Clindamycin Phosphate eq. to Clindamycin 1 % w/w.+ Nicotinamide IP 4 % w/w Gel |
| 15 | Clindamycin Phosphate IP EQ. TO Clindamycin 1 %w/w and Tretinoin USP 0.025%w/w Topical gel |
| 16 | Clindamycin Phosphate IP EQ. TO Clindamycin 1 %w/w and Tretinoin USP 0.05%w/w gel |
| 17 | Clobeatsol Propionate IP 0.05 %w/w + Ammonium Lactate 12 % w/w (Gel) |
| 18 | Clobetasol Propionate 0.025%w/w,Gentamicin sulphate eq. to Gentamicin 0.1%w/w, Miconazole Nitrate2.0%w/w,Zinc oxide3.0%w/w. Skin Lotion |
| 19 | Clobetasol Propionate 0.05%w/w,Gentamicin sulphate eq. to Gentamicin 0.2%w/w, Miconazole Nitrate2.0%w/w,Zinc oxide0.1%w/w. |
| 20 | Clobetasol propionate IP 0.05 %w/w (Clobetasol Cream IP) |
| 21 | Clobetasol propionate IP 0.05 %w/w, Neomycin Sulphate IP 0.50%w/w, Miconazole Nitrate IP 2.00%w/w,Chlorhexidine Gluconate Sol.IP 0.20%w/w |
| 22 | Clobetasol Propionate IP 0.05%w/w + Miconazole Nitrate IP 2.0%w/w + Neomycin Sulphate IP 0.5%w/w cream base chlorocresol IP 0.1%w/w as preservative. |
| 23 | Clobetasol Propionate IP 0.05%w/w and Salicylic Acid IP 6.0%w/w Ointment base |
| 24 | Clobetasol Propionate USP 0.05 % w/w, Miconazole Nitrate IP 2 % w/w Cream |
| 25 | Clobetasol Propionate USP 0.05 % w/w,Salicylic Acid IP 6 % w/w Cream |
| 26 | Clotrimazole cream IP 1.0% W/W |
| 27 | Clotrimazole cream IP 2.0% W/W |
| 28 | Clotrimazole IP 0.5%w/w + Menthol IP 1.0%w/w + Ichthammol USP0.2%w/w + Boric Acid IP 1.0%w/w + Zinc Oxide IP 5.0%w/w Preservative : Phenoyethanol IP 0.2%w/w and Benzyl Alcohol IP 1.0 %w/w |
| 29 | Clotrimazole IP 1.0%w/w and Beclomethasone Dipropionate IP 0.025%w/w Preservatives : Benzyl Alcohol IP1.0%w/w ,Methyl Paraben IP0.15%w/w Cream |
| 30 | Diclofenac Diethylamine(1.16%w/w) eq. to Diclofenac Sodium 1 % w/w |
| 31 | Diclofenac Diethylamine(1.16%w/w) eq. to Diclofenac Sodium 1 % w/w MethylSalicylate 10%w/w,Menthol 0.5 %w/w,Linseed Oil3 %w/w |
| 32 | Diclofenac Diethylamine(1.16%w/w) eq. to Diclofenac Sodium 1 % w/w MethylSalicylate 10%w/w,Menthol5 %w/w,Linseed Oil3 %w/w |
| 33 | Each gram contains : Fradiomycin Sulphate JP(Neomycin Sulphate IP) 5 mg Cream Chlorocresol IPas preservative 0.01%w/w |
| 34 | Each gram contains : Polymyxin B Sulphate USP Eq.to Polymyxin 5000 units Bacitracin Zinc IP EQ. to Bacitracin 400 units Neomycin Sulphate IP Eq. to Neomycin 3400 units Ointment Benzyl Alcohol IPas preservative 0.1%w/w |
| 35 | Flucinolone Acetonide IP 0.025 %w/w(Flucinolone Acetonide Cream IP ) |
| 36 | Fluticasone Cream IP (Fluticasone Propionate IP 0.05%w/w) |
| 37 | Fusidic Acid 2.0%w/w |
| 38 | Gamma Benzene Hexachloride IP 1%w/w, Cetrimide IP 0.1%w/w. Lotion. |
| 39 | Halobetasol propionate eq. to Halobetasol0.05 %w/w and Salicylic acid 6 % w/w cream |
| 40 | Halobetasol propionate USP eq. to Halobetasol0.05 %w/w (Halobetasol Propionate cream) |
| 41 | Heparin Sodium IP 50IU+ Benzyl Nicotinate 2mg + Sorbic Acid IP 1.97mg Ointment |
| 42 | Hydroquinone USP 2 %w/w, Tretinoin USP 0.025% w/w, Fluocinolone Acetonide IP 0.01 w/w CREAM |
| 43 | Hydroquinone 2 %w/w, Tretinoin 0.025% w/w, Mometasone Furoate 0.1 w/w |
| 44 | Hydroquinone cream USP (Hydroquinone USP 4 % w/w) |
| 45 | Itraconazole BP 1.0%w/w, Terbinafine Hydrochloride IP 1.0%w/w, cream |
| 46 | Ketoconazole 2 % w/w, Zinc Pyrithione 1 % w/w |
| 47 | Ketoconazole IP 2 % w/w (Ketoconazole Cream ) |
| 48 | Ketoconazole IP 2 % w/w (Ketoconazole LOTION ) |
| 49 | Ketoconazole IP2.0 %w/w and Beclomethasone Dipropionate IP 0.025%w/w Cream Preservatives : Methyl Paraben IP 0.15% w/w and PropylParaben IP 0.05%w/w |
| 50 | Luliconazole 1%w/w (Luliconazole cream) |
| 51 | Metronidazole IP 0.01 % w/w and Benzocaine IP 0.2 % w/w |
| 52 | Miconazole Nitrate gel 2 % w/w |
| 53 | Miconazole Nitrate IP 2 % w/w+ Nadifloxacin 1 % w/w and Mometasone Furoate IP 0.1%w/w cream |
| 54 | Minoxidil IP 5 % w/v (Minoxidil Topical Solution USP) |
| 55 | Mometasone Furoate 0.1 % w/w. |
| 56 | mometasone Furoate 0.1 % w/w. + Fusidic acid 2 %w/w |
| 57 | Mometasone FuroateIP 0.1%w/w+ Salicylic AcidIP%5%w/wOintment. |
| 58 | Mupirocin Cream USP 2 % W/W |
| 59 | Nadifloxacin USP 10 mg and Mometasone Furoate IP 1 mg cream |
| 60 | Octinoxate USP 7.5 %w/w+ Avobenzone USP 2.0 %w/w + OxybenzoneUSP 3.0%w/w+ Octocrylene USP 3.0%w/w+ Zinc OxideIP 2.0w/w Lotion |
| 61 | Ofloxacin IP 0.5 %w/w + Fluocinolone Acetonide IP 0.01%w/w + Clotrimazole IP 1 %w/w Preservative as Chlorocresol IP 0.1%w/w cream |
| 62 | Ofloxacin IP 0.75%w/w,Ornidazole IP 2.0%W/W,Itraconazole BP 1.0%w/w,Clobeatsol Propionate IP 0.05%w/w |
| 63 | OfloxacinIP0.75%w/w+ Ornidazole IP2%w/w+Terbinafine HydrochlorideBP1%w/w+Clobetasol PropionateIP 0.05% w/w Cream(BANNED FORMULA) |
| 64 | Permethrin 5 % w/w (Permethrin Cream) |
| 65 | Permethrin Lotion 5 % w/w |
| 66 | Potassium Nitrate BP 5 %w/w and Sodium Monofluorophosphate USP 0.7%w/w Toothpaste |
| 67 | Povidone Iodine IP 5 % w/w and Ornidazole IP 1 % w/w Ointment |
| 68 | Povidone Iodine IP 5 % w/w and SucralfateUSP 7 % w/w Ointment |
| 69 | Povidone- Iodine 5 %w/w |
| 70 | Povidone- Iodine 5 %w/w (Available Iodine 0.5 % w/w), Metronidazole 1 % w/w, Dexapanthenol 5 %w/w(YDIN-M OINTMENT) |
| 71 | Salicylic Acid Gel (Salicylic Acid IP 2.0%w/w) |
| 72 | Sertaconazole Nitrate 2% w/v + Zinc Pyrithione 1 % w/v |
| 73 | Sertaconazole Nitrate 2% w/v Cream |
| 74 | Silver Sulfadiazine USP 1.0 %w/w and IP Eq. to Chlorhexidine Gluconate 0.2 %w/w and Aloevera IP 15 %w/w and Allantoin IP 0.10%w/w Cream |
| 75 | Silver Sulphadiazine and Chlorhexidine Cream (Silver sulfadiazine IP 1.0%w/w and Chlorhexidine Gluconate solution IP EQ. TO Chlorhexidine Gluconate 5%w/w ) |
| 76 | Silver Sulphadiazine Cream IP 1.0 % w/w |
| 77 | Tacrolimus0.03%w/w Ointment |
| 78 | Terbinafine Hydrochloride BP Cream 1 % w/w |
| 79 | Tretinoin cream USP (Tretinoin USP 0.025 %w/w) |
| 80 | Tretinoin USPCream 0.05 % w/w |
| 81 | Urea IP 10%w/w and Lactic Acid IP 10%w/w and Propylene Glycol IP 10%w/w and Liquid Paraffin IP 10%w/w. Preservative : Methyl Paraben IP 0.16 %w/w and Propyl Paraben IP 0.04%w/w CREAM |
| 82 | White Soft Paraffin IP 13.2 %w/w+ Liquid Paraffin IP 10.2%w/w Cream |
| 83 | Zinc Oxide Cream IP 32% w/w |
List will be updated soon
List will be updated soon
List will be updated soon
Hygiene Care
List will be updated soon
List will be updated soon
List will be updated soon